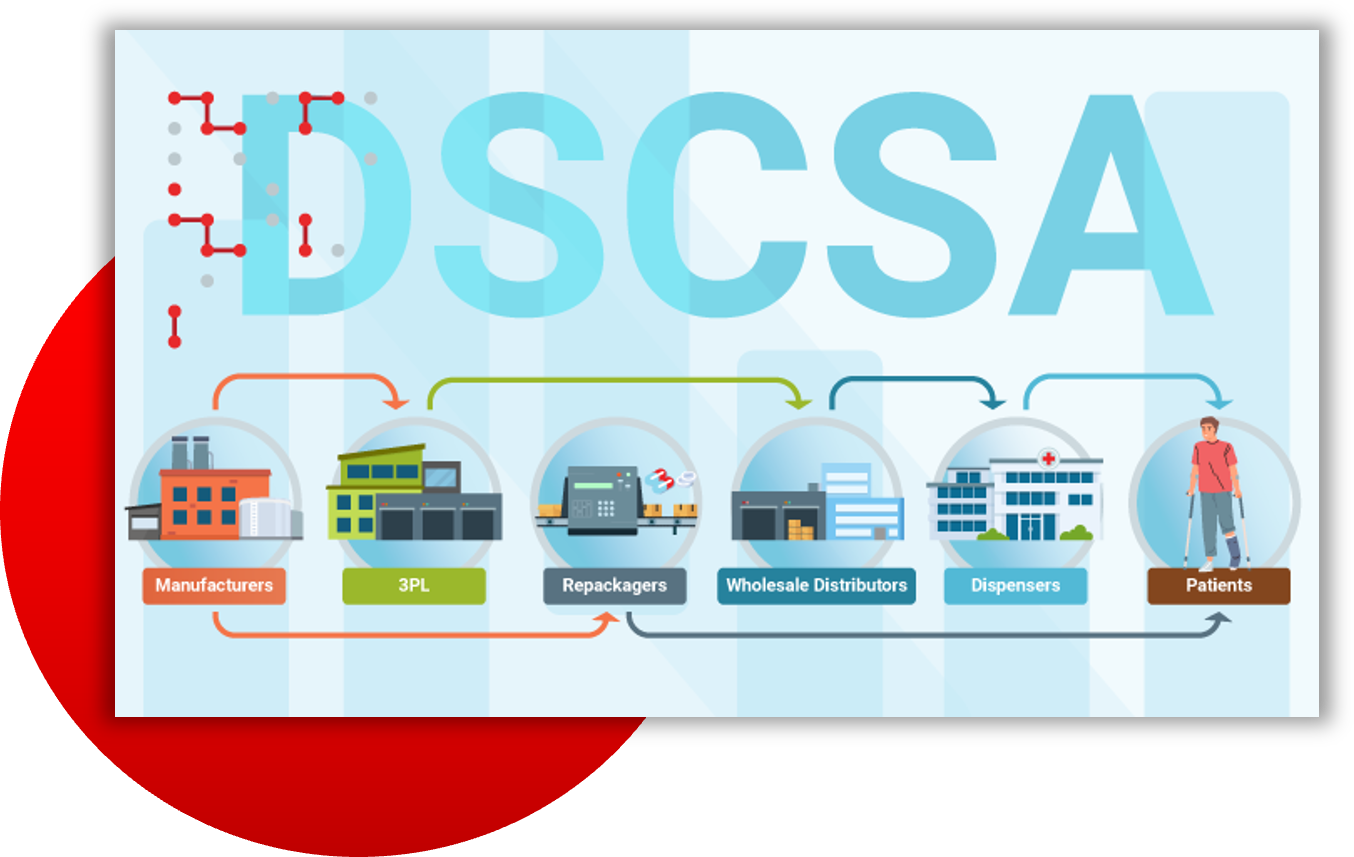
Infographic:
Your DSCSA Responsibilities
Discover essential guidelines for complying with the Drug Supply Chain Security Act (DSCSA). Learn how to enhance supply chain security and meet regulatory standards across various roles in the pharmaceutical industry.
Learning outcomes
- Identify specific responsibilities for manufacturers, 3PLs, repackagers, wholesale distributors and dispensers.
- Implement systems for verifying product identifiers and managing suspect items.
- Collect and record transaction data for full compliance and transparency.
- Standardize product labeling with NDC, serial numbers, lot numbers and expiration dates.
- Establish processes for investigating and quarantining suspect products.
- Set up systems for timely FDA notifications regarding suspect or illegitimate items.
Unlock the full potential of your compliance strategy. Download the infographic now to ensure your supply chain meets DSCSA requirements effectively.
Access Here

